Indications
Important Product InformationIndications
NeoVisc® HA 1.5% is indicated for treatment of pain and improvement of joint functionality in patients affected by degenerative (age-related changes) or mechanical arthropathy (related to overuse) of the knee.2
Do not use in case of package damage.
Do not use the product after the expiry date reported on the package.
The expiry date refers to the product kept in its original package at a temperature not exceeding 25° C.
The syringe is for single use, which means it should be used once only for a single patient. Inject the contents in one joint only.
For the first 24 hours after injection, the patient is permitted to continue all routine activities of daily living but is recommended not to overuse the treated joint.
The assembled syringe must be discarded immediately after use, regardless of whether the solution has been completely administered.
If this product is reprocessed and/or reused, Fidia Farmaceutici cannot guarantee performance, functionality, material structure, cleanliness or sterility of the product.
Reuse could lead to illness, infection and/or serious injury to the patient or user.
After use, dispose according to applicable national practice. Keep out of reach of children.
Contraindications2
Do not administer to patients with ascertained individual
hypersensitivity to the product components.
Do not administer in cases where there are infections or skin
diseases in the area of the injection site.
Do not administer to patients with active synovitis.
Dosage and Administration2
NeoVisc®+ 3 X 2 mL
Cycles of 2 mL at weekly intervals for 3 weeks.
NeoVisc® ONE 4 mL
Single intra-articular injection, may be repeated at or after 6 months.

What is NeoVisc?
NeoVisc is a gel-like substance manufactured from hyaluronic acid that is injected directly into your knee joint by your doctor. It is a naturally derived product made by biofermentation.
With knee OA, your knee joint fluid does not contain enough hyaluronic acid, which leads to pain and join stiffness. NeoVisc acts as a lubricant and a shock absorber in the knee joint, enabling it to work properly.2
NeoVisc relieves the pain of knee OA and improves mobility so you can get back to your favourite activities.3
Clinical Evidence
NeoVisc+ Improves Pain and Function3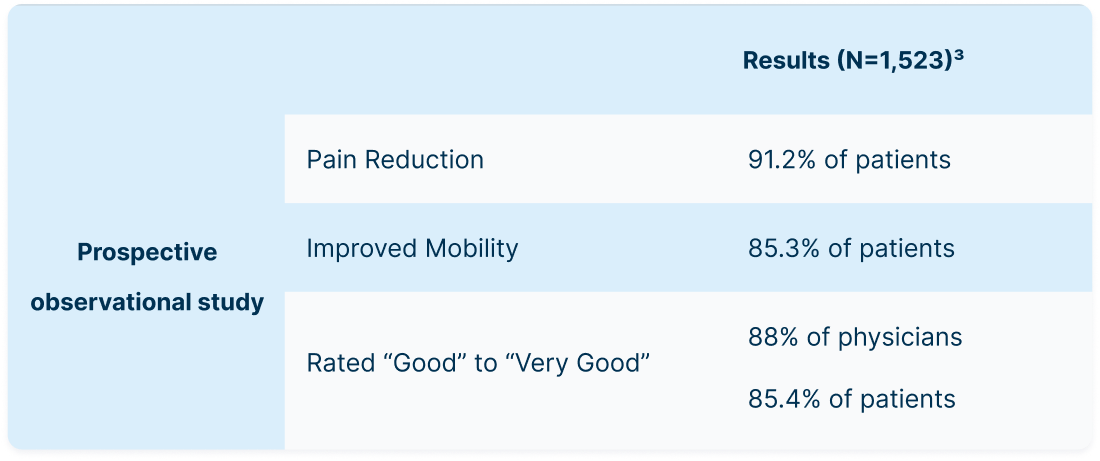
NeoVisc+ Reduces Pain Severity3
NeoVisc+ Reduces Pain on Movement4

NeoVisc ONE Reduces Knee Pain5

Results are from a cohort study in 168 patients with mild to moderate knee OA.5
*p<0.001

Information for Medical Professionals
References
1. Ojog S. Clinical evidence of knee osteoarthritis treatment with 2000 KDa HA up to 12 months. 4th International Symposium Intra Articular Treatment. (ISIAT) Budapest 1-3 October 2015.
2. NeoVisc®+ and NeoVisc® ONE Instructions for Use. September 2020.
3. Schieb F. Intra-articular injections of hyaluronic acid in the treatment of arthropathies. Arthritis & Rheuma. 2003;23:338-340.
4. Foti C, Cisari C, Carda S, et al. A prospective observational study of the clinical efficacy and safety of intra-articular sodium hyaluronate in synovial joints with osteoarthritis. Eur J Phys Rehabil Med. 2011;47:1-9.
5. Vetro A, et al. EJMD 2014; 3: 25-33.










